Describes an Atom That Has a Full Outermost Energy Level
Report an issue. An atom that has gained or lost electrons is called an.

The Joy Of Chemistry A Unit In Photos Scholastic Chemistry Classroom Science Chemistry Teaching Chemistry
Which of the following describes what happens when an atom becomes an ion with a 2- charge.
. An atom of helium has eight electrons in its outer energy. - describes an atom of the element helium. It has 6 valence electrons.
How many valence electrons are in a neutral atom of nitrogen N. The diagram below is the bohr model of an atom. It has a positive charge.
4s has lower energy than 3d. Elements created by a reaction. It has a positive charge.
An atom that has its outermost energy level filled is a noble gas. These are the answers. Which element has a full outermost energy level containing only two electrons.
It has 6 electrons. The electrons in an atoms outermost energy level. Helium atoms have 2 electrons while atoms of the other elements in the group all.
Because its outer shell is filled it is non reactive. The speed of a reaction. Helium atoms have a full outer energy level while atoms of the other elements in the group do not have a full outer energy level.
Orbital s has the lowest energy. Describes an atom that has a full outermost energy level. An atom of helium has eight electrons in its outer energy.
It will give up electrons forming a positive ion. A chemical bond formula is the force that holds atoms together in a compound. Two electrons is a full outer level because the first principal energy level only holds two electrons.
All the noble gases except helium have 18 8 electrons in their outer energy level. An outermost energy level that is full of electrons. In the progression thus far three atomshydrogen lithium and sodiumhave one electron in the outermost shell.
What charge does the resulting aluminum ion have. Atoms that lose or gain electrons are now_____and are called _____. An atom is chemically stable if its outer energy level __ _____ ____ electrons.
An tells what elements make up a compound and the ratios of the atoms of those elements. An atom of helium has a full outer energy level and is therefore unusually reactive. This shell has spaces for eight electrons so that it takes an atom with 10 electrons to fill the first two levels.
An atom is when its outer energy level is filled with electrons. Which best describes this atom. The electrons on the outermost energy level of the atom are called valence electrons.
What type of element tends to lose electrons when it forms bonds. A compound that has water chemically attached to its atoms and written into its chemical formula chemically stable describes an atom that has a full outermost energy level. An atom of helium most similarly behaves like an atom of hydrogen since it is in the same period.
It has 6 valence electrons. To obtain a full outer electron shell. Which of these does a balanced chemical equation show.
To fill the vacant energy levels the Aufbau Principle is. For atoms of most noble gases and most other elements the outer energy level os full when it has ____ electrons. An atom of helium has its valence electrons in its first energy level.
The next atom after neon sodium has 11 electrons so that one electron goes into the next highest shell. Molecule that has a slightly positive end and a slightly negative end. The atom gains 2 electrons.
Hydrogen has the stable electron configuration of the noble gas helium when it has two. The diagram below is the Bohr model of an atom. The number of electrons equal to the number of protons.
A molecule that has a positive end and a negative end is an molecule. For energy level 4 the placement of orbitals is 4s. It has a full outermost shell.
Which best describes this atom. It has 6 electrons. It has a full outermost energy level.
2 electrons in the first energy level. The reason why atoms combines is to have a complete outermost shell. Describes an atom that has a full outermost energy level.
An aluminum atom can lose 3 electrons in order to have a full outer energy level. Phase changes taking place during a reaction. An atom of helium has a full outer energy level and is therefore unusually reactive.
Helium atoms have a full outer energy level while atoms of the other elements in the group do not have a full outer energy level. Log in for more information. Which best describes this atom.
The attraction of each atoms nucleus for the valence electrons of the other atom pulls the atoms together. A chemical bond is formed when electrons are _____or exchanged to achieve a complete outer energy level. Describes an atom that has a full outermost energy level.
When the outermost shell or valence shell of atoms are completely filled withe electrons they become very stable. The valence electrons are involved in bonding one atom to another. The diagram below is the Bohr model of an atom.
Search for an answer or ask Weegy. It has a full outermost energy level. 8 electrons in the second energy level.
The outermost orbital of lower energy level has higher energy than the consequent orbital of higher energy level.

The Electrons In The Outermost Energy Level Of An Atom At Level

The Electrons In The Outermost Energy Level Of An Atom At Level
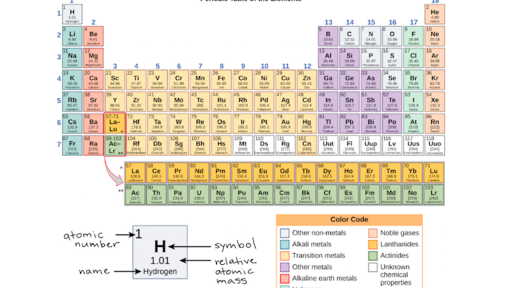
The Periodic Table Electron Shells And Orbitals Article Khan Academy

The Electrons In The Outermost Energy Level Of An Atom At Level

Image Result For What Is Valence Electrons 8th Grade Science Science Classroom

Subscripts Vs Coefficients 7th Grade Science 8th Grade Science Science Classroom

Atom Orbits And Energy Levels Britannica

The Electrons In The Outermost Energy Level Of An Atom At Level
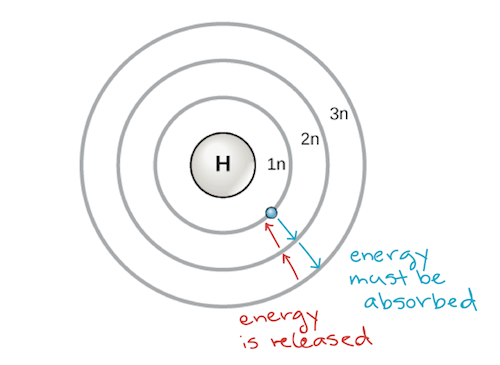
The Periodic Table Electron Shells And Orbitals Article Khan Academy

The Electrons In The Outermost Energy Level Of An Atom At Level

Unshared Pair Easy Science Easy Science Science Rules Science Facts

Valence Electrons Electrons Earth Science 8th Grade Science

The Atom Chemistry Is My Jam Atomic Theory John Dalton Atomic Theory Atom

Halogen Elements Examples Properties Uses Facts Britannica
What Is The Outermost Shell Of An Atom Called Quora
How Does The Arrangement Of Electrons In An Atom Affect The Chemical Properties Of The Elements Quora

What Are Ions The Chemistry Journey The Fuse School This Is An Excellent Review Of Atoms That Gain Or Lose E Teaching Science High School Science Chemistry
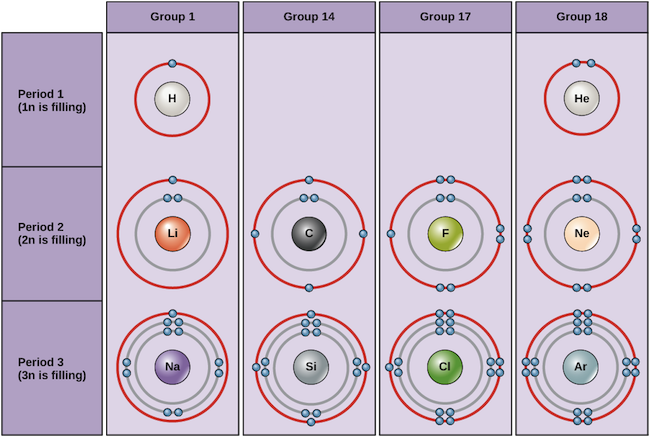
The Periodic Table Electron Shells And Orbitals Article Khan Academy

The Electrons In The Outermost Energy Level Of An Atom At Level

Comments
Post a Comment